Background
Congenital thrombotic thrombocytopenic purpura (cTTP) is an ultra-rare, life-threatening thrombotic microangiopathy caused by an inherited deficiency of the enzyme ADAMTS13. An ongoing phase 3 study (NCT03393975) and phase 3b continuation study (NCT04683003) are investigating the safety and efficacy of recombinant ADAMTS13 (rADAMTS13; TAK-755; Takeda Development Center Americas, Inc., Lexington, MA, USA) for prophylaxis and on-demand treatment of acute events in patients with cTTP.
Aims
To assess the efficacy and safety of rADAMTS13 for the treatment of acute TTP events and long-term prophylaxis in patients with severe cTTP.
Methods
The phase 3 prospective, randomized, controlled, open-label, multicenter, crossover study (NCT03393975) is open to enroll patients 0-70 years old with severe cTTP (ADAMTS13 activity <10%). Upon completion of the phase 3 study, patients could enroll into the phase 3b prospective, open-label, multicenter, single treatment arm continuation study (NCT04683003). In the phase 3 study, patients were randomized 1:1 at enrollment to receive prophylaxis with either rADAMTS13 or standard of care (SoC). SoC was determined by the treating physician and could include fresh frozen plasma, pooled solvent/detergent-treated plasma, or factor VIII/von Willebrand factor concentrates. Patients in the phase 3b continuation study received open-label rADAMTS13 treatment. In both studies, patients could be enrolled and receive treatment on demand for acute TTP events. Per protocol, rADAMTS13 was to be administered at 40 IU/kg on Day 1, 20 IU/kg on Day 2 and 15 IU/kg daily from Day 3 onwards until 2 days after resolution of the acute event (defined as platelet count either ≥150,000/μL or within 25% of baseline; and elevation of LDH levels ≤1.5× of baseline or ≤1.5× the upper limit of normal). Data are presented from a pre-planned interim analysis (data cutoff: August 12, 2022) and focus on the treatment of acute TTP events in both studies.
Results
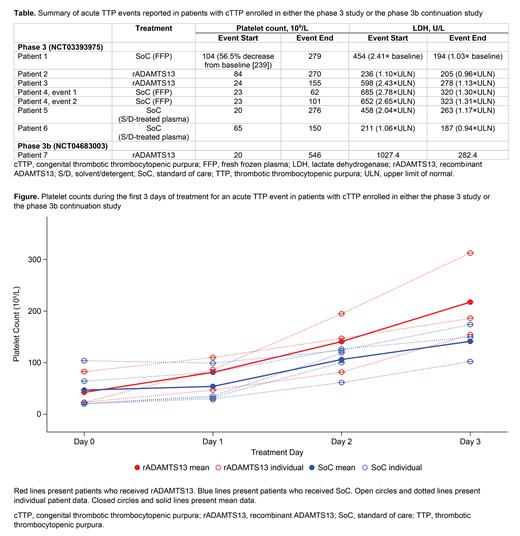
At the time of the data cutoff, 8 acute TTP events were reported in 7 patients ( Table), all of whom were ≥18 years old. None of the events occurred while the patient was receiving rADAMTS13 prophylaxis. Six events occurred in 5 patients (median age: 20.0 years; sex: 3 male, 2 female) who received on-demand treatment in the phase 3 study; 2 of these patients were randomized to receive rADAMTS13 and 3 to receive SoC. One event also occurred in the phase 3 study while the patient was receiving prophylaxis with SoC. One event occurred in the screening period prior to the patient starting rADAMTS13 prophylaxis in the phase 3b continuation study; this event was treated with rADAMTS13. Platelet counts observed during the first 3 days of treatment for all 8 acute TTP events are shown in the Figure. Prior to study treatment, on Day 0, the mean platelet counts were similar between the rADAMTS13 and SoC cohorts. Over the first 3 treatment days, the mean platelet count increased more than ~5-fold with rADAMTS13, and ~3-fold with SoC. All three acute TTP events treated with rADAMTS13 resolved without requiring the administration of any other treatment containing ADAMTS13. Based on pharmacokinetics (PK) data available in 5 patients (3 received treatment with rADAMTS13 and 2 with SoC), the post-infusion ADAMTS13 activity levels ~1 hour after administration were between 80% to 270% of normal with rADAMTS13 and between 10% to 48% with SoC. These observed ADAMTS13 activity levels were as expected based on the linear PK observed following single dose PK assessments in the phase 3 study. Of the 4 patients who received SoC for the treatment of an acute TTP event, 2 experienced a mild treatment-emergent adverse event (TEAE) that was considered related to the SoC (nausea and pruritis). Of the 3 patients who received rADAMTS13, 1 patient experienced 3 mild TEAEs (feeling hot, nausea, and thrombocytosis) that were considered related to rADAMTS13. No serious TEAEs related to rADAMTS13 were reported and no neutralizing antibodies against ADAMTS13 were observed.
Conclusions
The resolution of acute TTP events and an improvement in platelet count in patients with cTTP following treatment with rADAMTS13 was closely related to higher ADAMTS13 activity exposure. No serious TEAEs related to rADAMTS13 were reported.
Disclosures
Scully:Octopharma: Speakers Bureau; Alexion: Research Funding; Takeda: Honoraria, Research Funding, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau. Ortel:Takeda: Research Funding; UpToDate: Honoraria; Siemens: Research Funding; Stago: Research Funding; Sanofi: Consultancy; CDC: Research Funding; NIH: Research Funding; Instrumentation Laboratory: Consultancy, Research Funding. Waliullah:Takeda Development Center Americas Inc.: Other: Contractor. Zhang:Takeda Development Center Americas Inc.: Current Employment, Current holder of stock options in a privately-held company. Patel:Takeda Development Center Americas Inc.: Current Employment, Current holder of stock options in a privately-held company. Patwari:Takeda Development Center Americas, Inc.: Current Employment, Current holder of stock options in a privately-held company. Mellgard:Takeda Development Center Americas, Inc.: Current Employment, Current holder of stock options in a privately-held company. Wang:Takeda Development Center Americas, Inc.: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal